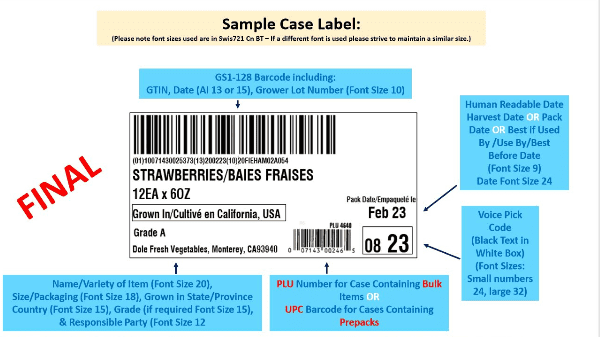
Just a few weeks after releasing its improved traceability guidance, the Produce Traceability Initiative is encouraging buying groups to join the 15 who have already shown support for a harmonized case label.
The companies who have officially shown support and sent vendors letters requesting its use are:
- Albertsons Companies BB #:193326
- Aldi, Inc. BB #:116756
- Buy Low Foods BB #:116378
- Costco Wholesale Canada BB #:152593 and US BB #:150902
- Federated Co-operatives BB #:150831
- Loblaw Companies Limited BB #:138169
- Markon BB #:123315
- Metro BB #:115890
- Publix BB #:110909
- Sam’s Club BB #:140368
- Sobeys BB #:116615
- Sysco Corporation Canada BB #:157044 and US BB #:105962
- Walmart Canada
- Walmart US BB #:143789
- Wegmans BB #:104173
In a round table meeting during the Produce Marketing Association’s BB #:153708 weekly town hall webinar July 15, PTI leaders discussed other next steps.
Ed Treacy, vice president of supply chain and sustainability for PMA, also noted a multi-association initiative to test a traceback template through an industry pilot to see if it helps and enables data sharing with FDA, with the goal of having results before the next group of Food Safety Modernization Act deadlines. The group plans release more details next week.
Katie Vierk, director of the FDA division of public health informatics and analytics, said her group plans to send a rule for comments by September 8.
More information about PTI’s latest steps and the traceback template are on its website.
CORRECTION: An earlier version of this story misstated the details of the pilot program.